- Ongoing research projects
- CCBN animal housing and animal care facility
- Animal surgical facility
- Parallel computing facility
- Optogenetics
- In situ hybridization/IEG lab
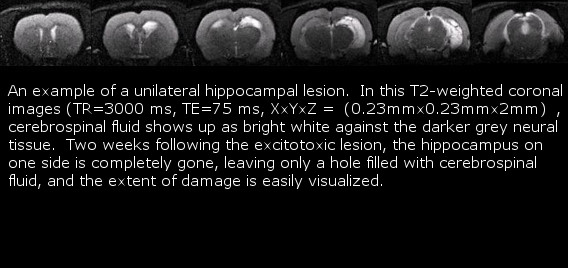
- CCBN Small Animal Magnetic resonance Imaging Facility
- Polaris imaging facility
- Voltage sensitive dye recording
- Microendoscope in-vivo imaging
- 2-photon microscopy
- Modular 2-photon microscopy
- Transmission electron microscopy
- In vitro electrophysiology
- Acute electrophysiology using silicon probes
- Neural ensemble recording in behaving animals
- Workshop facilities
- Electrode & microdrive assembly room
- Electronics
- Animal behaviour facilities
- 6-Animal electrophysiology facility
- Immunohistochemistry protocols
Ongoing research projects
Ongoing research projects in the Brain Dynamics group include the following:
- Spatial orientation mechanisms (grid cells, place cells, head direction cells)
- Offline reactivation of memory traces
- The role of sleep and memory consolidation
- The role of the hippocampus in information processing
- Interactions between hippocampus and neocortex
- The role of the prefrontal cortex in decision making
- The role of neuromodulators for learning and information processing in cortex-basal ganglia circuits
- Altered neural processing in psychiatric illnesses using pre-clinical (rodent) models
- Addiction
- Sound processing by cortical circuits in animal models of stroke, MS, and hippocampal lesions
- Brain plasticity and motor control
- Brain Aging
- Brain/computer interface
- Development of new mathematical tools for the analysis of brain dynamics, particularly for large-scale neural recordings
- Neural network models of higher cognitive function
CCBN animal housing and animal care facility
Animal Care Staff
- Karen Dow-Cazal
- Moira Holley
- Charlotte Holmes
- James Cazal
- Janine Brewster-Higgins
- Carla Navratil
Veterinarian
- Isabelle Gauthier, DVM
Animal surgical facility
The surgical suite in the Canadian Centre for Behavioural Neuroscience (CCBN) offers an aseptic environment for researchers to conduct various types of neurological surgeries such as the electrode array implanting (a.k.a. hyperdrives). The CCBN has three state of the art operating rooms: one large multi-purpose operation room and two small rooms. The large room has sufficient space where the researchers can bring their own peripheral equipment for the procedure. All operating rooms provide an advanced surgical environment under sterilized conditions and are equipped with a ventilation system, anaesthetic equipment, operating table, surgical microscope, surgical light, bench space, and a sink.
The CCBN also has a surgical preparation room in order to prepare for various surgical procedures. Sterilized surgical supplies such as drapes, solutions and various instruments are available in this room. An autoclave is available for sterilization of all surgical instruments. The room also serves as an animal recovery area; several medications, heating pad, and two types of animal bedding are available for post-operative treatment. After any surgery, animals are allowed to recover for several hours in this quiet room, and are carefully monitored and administered analgesics.
Parallel computing facility
Hodgkin, our computing cluster, has 64 blade servers containing 48 GB of RAM and two 6-core Intel Xeon processors. When added up, Hodgkin has 768 available computing cores and 3 TB (3072 GB) of RAM for use. Each of the blade servers are running 64-bit Red Hat Enterprise Linux.
The scheduler used on Hodgkin is IBM Platform LSF, which is officially supported by MATLAB. Hodgkin is currently configured to use the most current release of MATLAB (R2012b), and some older versions are available as well (R2010a, R2010b, R2011a, R2011b and R2012a). MATLAB is currently the primary use of Hodgkin.
For our storage cluster, Huxley, we have 50 TB of hard drive space. This space is split up into two different 16TB volumes based on the type of project, with an extra 10 TB volume for general use. There is 5TB of scratch space for temporary files.
The contents of Huxley are backed up every night to an off-site location. The off-site location has 80TB of storage which allows us to have incremental backups. Data is backed up here for 6 months. Snapshots are kept for the most recent seven days, three weeks, and six months.
Current research being done using the parallel computing facility includes speeding up spike sorting preprocessing (Klustakwik), analysis of spike train data by template matching, calculating cross correlations between neural spikes, simulation of Hopfield-type neural networks, and IEG image analysis. By taking advantage of a large number of available cores, a large amount of RAM and MATLAB’s parallel computing toolbox, significant speed up of existing code (up to ~700 times) has been achieved.
The Polaris group also uses GPUs to help speed up the IEG image analysis and perform neural network simulations using the Izhikevich model, which has sped up the calculations by more than 100 times.
Scientific computing in the Polaris group is supported by three full time programmer/administrators who develop new analysis code and assist personnel with computing needs.
Optogenetics
Optogenetics is a recently-developed technology for rapidly and selectively controlling neural activity with light. We have constructed a laboratory to use this technology in behaving animals and in acute brain slices. The lab contains a new Containment Level 2 (CL-2) surgical suite for injection of viral-based vectors to express light-sensitive proteins in neurons, and an in vivo recording/optogenetics setup that allows us to record 96 channels of electrophysiological data from behaving animals while also delivering light for optogenetic manipulation. We have also expanded our in vitro rig to allow optogenetic manipulation in acute brain slices.
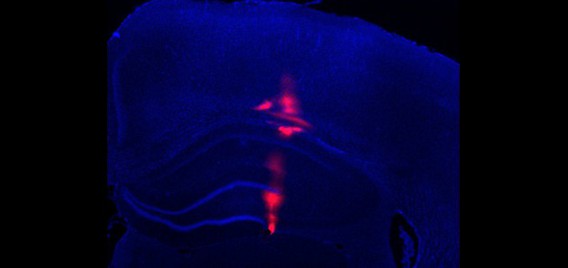
The in situ lab
Previously, the In Situ Lab at the Canadian Center for Behavioural Neuroscience (CCBN) was far too small for the needs of the expanding Polaris Brain Dynamics Research Group. Funding has allowed the much needed expansion of the CCBN building to include a much larger and better equipped In Situ facility to support the work of the growing Polaris team as well as other research groups within the building.
The new facility has a vastly expanded workspace and is furnished with two large fume hoods as well as a laminar flow hood for sensitive applications. The lab includes heating and cooling equipment such as 4oC fridges, a -20oC freezer, an ultralow -80oC freezer as well as two hybridization ovens and an economy oven. The room itself is equipped with HEPA-filtered ventilation, washable ceiling tiles and positive pressure to reduce dust and contaminants. An adjoining reagent and specimen room increases the storage capacity of the main In Situ room. The In Situ Lab is also supplied with numerous amenities such as Reverse Osmosis (RO) water, a Nanopure Diamond filtration system, a Nanodrop 2000 spectrophotometer, a dishwasher, emergency safety equipment (emergency shower, eye wash station and first aid kit) and two large double sinks.
The expanded In Situ Lab is capable of supporting multiple workstations simultaneously and two Research Technicians are primarily responsible for assisting undergraduate students, graduate students, post-doctoral fellows and principal investigators in their research. The lab space is principally used for Fluorescent In Situ Hybridization (FISH), a sensitive process that allows the detection and visualization of mRNA on tissue sections. The lab is also used for reagent and tissue preparation and is equipped to support other molecular biology processes including PCR and riboprobe synthesis (which are essential to the FISH process). The facility is also expanding to support quantitative real-time PCR (qRT-PCR), a process that enables simultaneous detection and quantification of gene expression, and the lab currently accommodates the associated RNA extractions, cDNA synthesis and other related processes.
CBN Small Animal Magnetic resonance Imaging Facility
Polaris members have access to the CCBN small animal MRI facility. Current use includes in vivo analysis of brain lesions to screen subjects for behavioural and immediate-early gene expression studies. The facility is maintained by Dr. Albert Cross’s lab who also assist will all scanning. The 4.7T magnet is capable of scanning at a resolution of 100 microns, and can acquire either serial 2-D images or a full 3-D scan. Slices within the volume of interest can be easily positioned to investigate specific regions of the brain. Using a non-magnetic stereotaxic holder, the anesthetized animal may be scanned repeatedly over time to track lesion development or contrast dye diffusion and uptake.
Polaris imaging facility
The Polaris Imaging Facility features two state-of-the-art NanoZoomer Digital Pathology (NDP) systems (Olympus & Hamamatsu) and an Olympus FV1000 spectral confocal system, which serve as powerful tools in neuroscientific research in brain circuitry and neural networks. The NanoZoomer systems are capable of rapid and automated scanning of whole-brain images in fluorescence and/or bright field. In addition, each system can scan up to six individual slides in manual or automated batch mode. The option of fully automated batch processing maintains consistent focus information. Depending on section thickness or quantification parameters, researchers may choose to scan tissue samples in multiple layers up to 21 z- focal planes, a feature which enables detailed 3D reconstruction. NanoZoomer systems are capable of visualizing samples labeled with multiple fluorescent immediate-early gene markers and/or fluorescent neuroanatomical tract tracers. The high resolution and large-scale visualization features allow researchers to focus on whole-brain sections or to zoom into specific regions such as hippocampal subregions. These images are stored in a compressed digital format that permits mass storage without sacrificing image integrity or resolution by employing millions of pixel resolution. This also allows for a diverse array of zoom and magnification scales during review processes. These NanoZoomer systems are complemented with a diverse suite of practical software for image analysis and quantification. The NDPView application enables simultaneous viewing of multiple images for easy cross-regional and cross-sectional comparison. Capitalizing on Hamamatsu’s advanced sensor technology, scans are fast, reliable, and consistent. These features culminate to provide high data throughput abilities for the tracking of neuronal network activity and brain circuitry dynamics.
The Olympus confocal system was purchased through a Canadian Foundation for Innovation (CFI) grant and installed in August, 2011. It includes a BX81 upright microscope with 4X, X10, X40 oil, and X60 oil lenses. All lenses are high N.A. plan apochromats with DIC optics. The lasers are all diodes with lines at 405nm, 473nm, 559nm and 635nm. Fluorescent filters include DAPI, FITC, Tx-Red and Cy5 mounted on a motorized turret. Four PMT detectors, including two spectral and two filter-based, enable high-precision (1nm steps up to 100nm), high-resolution (2nm bandwidth), high-speed (100nm/ms) spectroscopy. The spectral scan unit is capable of capturing 16 frames per second at a resolution of 256 X 256 pixels or 4 frames per second at 512 X 512 pixels. This instrument is the only confocal to date that offers two means of spectral un-mixing through “normal” and “blind” deconvolution. The advantage of a confocal microscope over a conventional microscope is the ability to optically section samples by virtue of the confocal pinhole. This aperture is located in the light path in close proximity to the detectors and serves to remove out-of-focus illumination and thus capture thin (10 nm) optical slices in an image stack that can be rendered into 3-D movies (see associated videos). The spectral confocal is a facility workhorse and is used for a multitude of functions mostly related to imaging immediate-early gene and neuroanatomical tract tracing products.
Voltage sensitive dye imaging
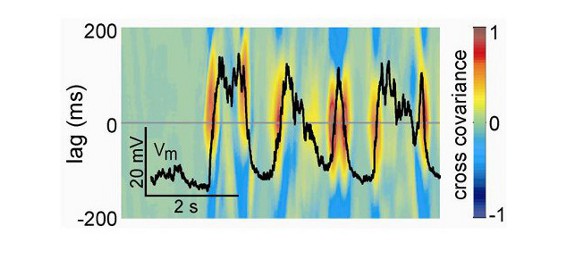
Voltage sensitive dye imaging (VSDI) allows direct recording of cortical activity within large neuronal populations (>10mm) with high spatial (down to 20-50 µm) and temporal (down to the millisecond) resolution. This technique is based on the use of a dye (e.g. RH1691 – Optical Imaging) that binds to cell membranes and acts as a local probe indicating the level of depolarization by an emission of fluorescent photons. This fluorescent signal mainly reflects dendritic activity of excitatory neurons in superficial layers.
Voltage-sensitive dye signals are small and require a high sensitivity and high speed imaging system. The lab is equipped with a MiCAM ULTIMA CMOS camera (SciMedia) which can achieve a high-speed image acquisition rate of up to 10,000 frames per second. The camera was mounted at the top of an Olympus MVX10 Macro Zoom System microscope equipped with epifluorescence, and a 100W halogen lamp gated by a shutter (Uniblitz) provides excitation light.
VSDI combined with electrophysiological techniques (e.g. EEG or high density electrode arrays) is a powerful tool for studying large neuronal ensemble dynamics with the highest spatiotemporal resolution nowadays available.
Currently, we are simultaneously performing VSDI and electrophysiological recordings in vivo in adult and developing rats.
Microendoscope in-vivo imaging
Complementary to electrophysiological studies, a more recent method of studying the activity of cells within the brain involves imaging genetically encoded calcium indicators (GECIs) expressed in target cells within the brain. Membrane bound GECI proteins emit fluorescent signals proportional to changes in intracellular calcium concentration. These signals thus provide insight into cell polarity and can be monitored simply by imaging the fluorescent signal emitted. GECIs require excitation with wavelength specific light to acquire the emitted fluorescent signal. In the past, these types of studies were restricted to use in applications where the head is fixed in place under the objective of a microscope. Advances in LED light for GECI excitation and photon detector technology has allowed the development of miniature head mountable microscopes which can be transiently affixed to a mouse’s head and subsequently image cell activity in awake freely roaming behaviours excluding the need for head fixation. Deeper structures can be imaged using implantable lenses allowing imaging of cell activity in deep structures. This technology has significantly increased the yield of cell activity per sample over electrode implantation and is a fast growing area in neuroscience. We have also successfully applied this technology to use within a virtual reality environment. To date, these micro-endoscopes are used in mouse experiments but will be applied to rat studies in the near future. This technology keeps us at the forefront of imaging based neuroscience research.
2-photon microscopy
The Olympus FV1000 MPE 2-photon microscope was purchased through a Canadian Foundation for Innovation (CFI) grant and installed in November, 2011. The scope is an Olympus BX61WI upright with two water-immersion lenses, a X10, N.A. 0.6 and a X25, N.A. 1.05, both equipped for DIC imaging. Both lenses were specially designed for 2-photon microscopy. Four high-efficiency non-descanned detectors provide efficient capture of the back-scattered fluorescent signal. For compatibility with the other facilities microscopy equipment the fluorescent filters are DAPI, FITC, Tx-red and Cy5. The stage is a Siskiyou 25mm XY translation platform and is large enough to hold electrophysiology equipment. Since this is a dedicated 2-photon instrument, only a single IR laser, a Spectra-Physics Mai Tai DeepSee, is included. The laser is tunable from 690nm – 1040nm. Group Velocity Dispersion (GVD) and laser beam auto-expander provide beam dispersion compensation and back aperture illumination optimization.
The advantage of a 2-photon system over a confocal microscope is the enhanced ability to image deeply into a specimen with a minimum of radiation damage and fluorescence quenching. The instrument accomplishes this by projecting a femtosecond pulsed infared beam into the specimen to excite fluorescent dyes.
The 2-Photon scope will be used in a number of ways, including serial section block face imaging, categorizing neuronal cells into classes and the analysis of in-vivo neuronal spine dynamics. Block face imaging will be accomplished through the use of a specially designed vibratome that will reside on the mechanical stage. Neurons will be classified using “Brainbow” mice in conjunction with immediate-early gene product imaging. Spine dynamics will be investigated through anaesthetised cranial windows accessing deep cortical layers.
Modular 2-photon microscopy
In addition to the package system provided from known microscopy companies, modular customisable 2-Photon microscopy systems are also available to allow the user to focus imaging systems toward more specific tasks. The advantage of custom assembled systems is their flexibility for future upgrades and modifications. We have two such systems of high specification and imaging ability.
In both the Sutter MOM® (Movable Objective Microscope®) and Thorlabs Bergamo II 2-Photon systems, the objective and microscope move over the sample instead of the sample moving on a stage beneath the objective as in most package systems. This is particularly advantageous when performing in-vivo imaging of awake specimens allowing Z-stacks and mosaic images of large regions of tissue in samples where moving stages are not suitable.
Both systems are supplied with two Ti:Sapphire Chameleon Ultra II lasers. These lasers have the highest output commercially available (up to ›3.5W) and widest tuning range of up to 400nm (680 to 1080nm).
Both lasers entering the MOM systems at different wavelengths allows simultaneous imaging of two fluorophores in a single sample. Scanning is done by galvanometer (galvo) mirrors and emission from the sample is detected by two Hamamatsu PMTs.
The Bergamo II has dual scan paths; one with galvo-galvo scanning and one with galvo-resonant scanning. Galvo-resonant scanning is the fastest scanning method widely available. Using this method, frame rates of 30fps at 512 x 512 and 400fps at 512 x 32 pixels is achievable. With dual beam paths, the Bergamo II allows imaging with a resonant scanning pathway while simultaneous stimulation of the sample with the galvo scanner. Stimulation can be localized to small and/or multiple areas allowing precise control. Z-axis objective positioning is done using high precision motors with a resolution of 0.1µm. Z-axis scanning however, is performed with a piezo objective holder providing a much faster z-axis shift and 2.5nm resolution.
The reflected emission signal is picked up by two GaAsP (Gallium Arsenide Phosphide) PMTs with high quantum efficiency. Epifluorescence is supplied by a multi-wavelength high power LED source through a liquid light guide.
Features for beam conditioning include, variable attenuators, variable expanders and the use of Pockel cells for sample exposure control.
The versatility of the modular systems allows them to be applied to almost any experimental protocol. Imaging of the brain in awake rodent models can be performed in addition to supplementary data acquisition methods such as electrophysiological recording with probes or patch clamp.
Transmission electron microscopy
The Hitachi H-7500 TEM was purchased in 2000 through a Natural Sciences and Engineering Research Council of Canada grant. It is a standard research TEM capable of 2nm resolution (theoretical = 0.2nm) and has a maximum accelerating voltage of 125K. The scope is equipped with a SIA-L11C digital camera with 16 megapixel resolution and the operating system has been upgraded to Windows XP. The stage is a goniometer enabling the production of 3-D images.
Because an electron microscope uses electrons rather than photons for imaging, the resolution limit is approximately 1000 times lower than what can be achieved with a light microscope. In addition, due to the small apertures employed, the depth of focus and depth of field are significantly greater than that of a light microscope.
The primary use of the scope is to image thin sections of rat neural tissue but it has also been used for several other procedures including colloidal gold labeling and negative staining.
In vitro electrophysiology
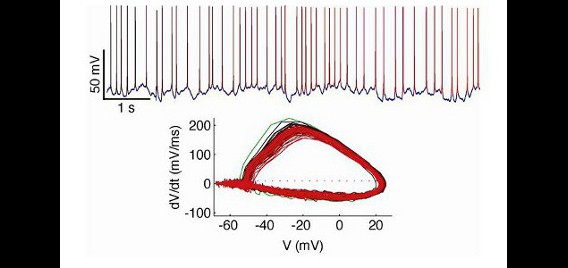
Our in vitro electrophysiology lab contains a full suite of equipment for preparing live sections of brain tissue and performing intracellular recordings using patch-clamp techniques under visual guidance. The recording rig is based on an Olympus BX51-WI microscope with IR-DIC components and an IR-capable camera. Electrodes are fabricated on-site with a Sutter P-97 Flaming/Brown micropipette puller, and are positioned in the work area with two Sutter MP-225 micromanipulators. An Axon 700B amplifier and Digidata 1440A data acquisition system are used for data collection. For stimulus presentation, we have built a custom real-time control system based on a real-time linux workstation running RTXI. This allows real-time feedback to recorded neurons, permitting a full complement of ‘dynamic clamp’ techniques. For instance, with this rig we are able to simulate and insert fictive conductances into real neurons to assess how biophysical properties of the neural membrane contribute to excitability. We also use this rig to record the response of neurons in slice preparations to complex inputs that mimic time-varying features of neurons recorded in intact brains.
Acute electrophysiology using silicon probes
Neural ensemble recording in behaving animals
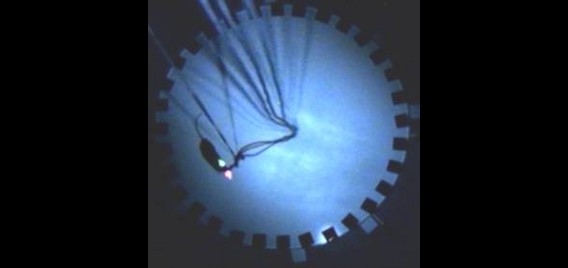
The Polaris group is equipped with extensive facilities for recording brain activity in behaving rodents. These facilities consist of five neural ensemble recording studios, each of which are made up of a control room housing the amplifiers and computers required for acquisition of the electrophysiological signals, and an experimental room housing the apparatuses on which the animals are tested. In each experimental room, the chronically implanted electrodes of the experimental subject are plugged into cables connected to a ceiling-mounted automatically rotating commutator, allowing the animal to rotate freely without twisting the cables; the commutator is in turn connected to the amplifiers by way of cables running through the ceiling into the adjoining control room. Video of the animal is simultaneously recorded in order to track behaviour. Additionally, one of the five studios is equipped for simultaneous drug delivery.
Each recording studio contains a digital recording system consisting of amplifiers, analog to digital converters so that data can be recorded to computer, and recording software through which amplifier parameters can be controlled. Currently, each of our systems allow for simultaneous recording from 128 electrode channels, which represents a practical limit imposed by current electrode technology; however, all systems are expandable to 256 channels. Each of these channels can be used both for recording local field potentials as well as the spiking activity of individual neurons. Typically, groups of four electrodes are twisted together to make what is called a tetrode, which can record spikes from up to 10-15 neurons simultaneously. Using a process akin to the triangulation used by radar systems to locate objects, we can determine how many cells a given tetrode is recording from and, more importantly, we can determine which of those cells any given spike is emanating from. Thus, using all 128 electrode channels, we are able to record from hundreds of individual neurons in a single animal.
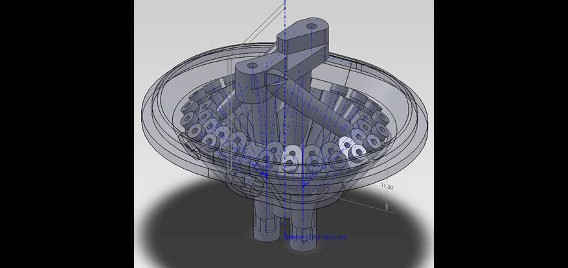
Rats are implanted with electrode arrays called hyperdrives which permit fine tuning of electrode position, allowing targeted isolation of the neurons of interest. We custom design our hyperdrives using 3D-computer aided design (CAD) software, which are then produced using stereolighography (3D plastic printing) technology. These custom designed drives allow flexible targeting of multiple brain regions simultaneously, greatly increasing the sophistication of our studies.
Each recording studio has a separate system for controlling and delivering precisely timed rewards and stimuli, including brain stimulation reinforcement which permits the use of complex behavioural paradigms that would not be possible with conventional food reinforcement. These control systems can be programmed to either respond to keyboard input from the experimenter or to be triggered by specific behavioural or electrophysiological events recorded by the acquisition hardware, providing us a tremendous amount of flexibility in designing our experiments.
Workshop facilities
The Polaris group has a dedicated workshop used for fabrication and manufacturing of experimental devices and project needs. It is outfitted with a variety of tools for metal and wood work as well as for the manufacture and repair of electronic devices.
Equipment:
- Small metal lathe 7″ x 12″ capacity
- Bench top milling machine bed dimension 15-3/8″ x 3-5/8″
- CNC Sherline Milling Machine with 4 axis control
- SolidWorks Cad software. Used in design and development of parts
- Diamond blade cutting tool
- Table Saw for wood cutting
- Miter chop saw
- Soldering station with fume extraction
- Bench top space
- Tool cabinet with selection of hand tools
- Shop vac
- Bench Vice
- Heat gun
Electrode & microdrive assembly room
The electrode and microdrive assembly room provides a clean environment for final assembly and preparation of hyperdrives. It includes equipment for creation of ground and stimulation electrodes, as well as for a final cut and gold plating of tetrodes.
Equipment:
- Dissection scope
- Gold plating equipment
- Carbon tipped serrated scissors
- Wire
- Impedence meter
- Heat gun
- Various clamps for holding microdrives
Electronic capacities
The Polaris group develops its own electronic circuits to adapt its data recording system to get reliability and good quality. These electronics (tetrode probe, silicon probe, single electrode) are composed to process data from the brain and manage stimuli, reward delivery and several controls (visual, auditory and electrical stimuli).
The Polaris group also develops Printed Circuit Boards (PCB) such as:
- Electrode Interface Boards (EIB) which adapt different kinds of probes to the Head Stage.
- Electronic circuits which convert high amplitude signals to the Transistor–transistor logic (TTL), triggering events, and amplification.
For some developments, Polaris researchers are using an open-source electronic prototyping platform based on flexible, easy-to-use hardware and software (http://www.arduino.cc). For other needs, the group is using a Field-Programmable Gate Array (FPGA). These systems allow researchers to manage data with good accuracy and a lot of flexibility.
Prototyping circuits are done with spice modeling (http://www.5spice.com). All the PCB are developed on Eaglecad (http://www.cadsoftusa.com) and are ordered from several PCB companies (ExpressPCB, http://www.expresspcb.com ; JetPCB , http://www.jetpcbassembly.com ; Silvercircuits, http://www.custompcb.com). All designs are imported into Computer-Aided Design (CAD) software (http://www.solidworks.com) to avoid mechanical conflicts with other parts. All the electronic parts come from electronic providers such as DigiKey (http://www.digikey.com), Mouser Electronics (http://www.mouser.com), or Robotshop (http://www.robotshop.com).
Animal behaviour facilities
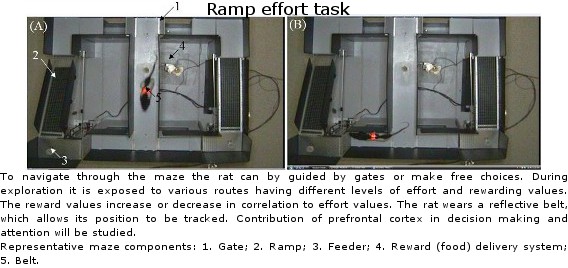
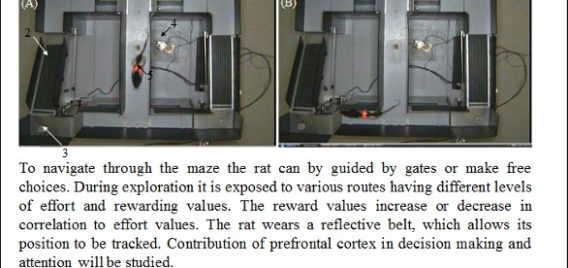
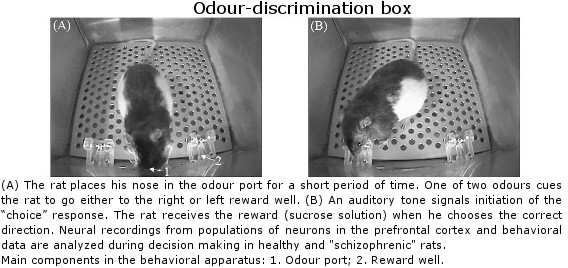
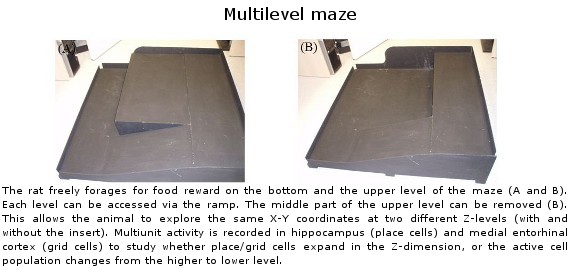
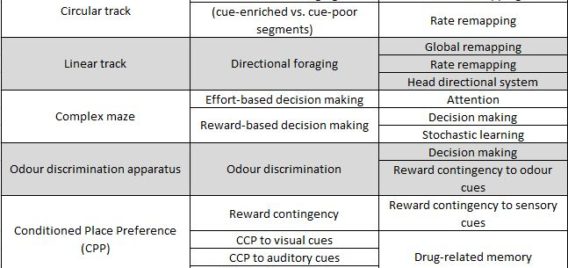
Lethbridge Brain Dynamics provides a variety of behavioral testing facilities to study neurophysiological and molecular mechanisms of spatial navigation, learning and memory in rats and mice. Behavioral paradigms/tasks are designed to investigate brain functioning during development, normal and pathological aging, and in various neurodegenerative diseases (e.g., Alzheimer’s disease) and in animal models for neurological disorders (e.g., stroke, schizophrenia, drug addiction, epilepsy). The rodent’s behavior can be tracked and/or filmed using both commercially available and custom designed tracking systems, and high-speed and conventional video systems, respectively. Most of the tasks are computer-controlled (commercial and home-written special algorithms) that enable both on-line and off-line analysis of the animal’s behavior and/or task modification. The behavioural tasks are ideally suited for electrophysiological (large-scale parallel neural recording), pharmacological (permanent or temporal inactivation of brain regions) and/or molecular imaging (large scale mapping of behavior-associated immediate early genes) studies in freely behaving animals. Available paradigms and tasks are outlined in the following table.

6-Animal electrophysiology facility
Our hardware design allows us to simultaneously record local field potential activity from six animals continuously with excellent stability for many months. Each recording unit has been outfitted with an Avatar EEG recorder, which is equipped with multiplexed Bluetooth technology allowing for the wireless transmission of recorded data. The Avatar EEG Recorder operates with a 256Hz sampling rate, bandpass filtering at 0.1Hz-100Hz and a range of +/- 6.5mV. Neurotek headstages and power supplies are employed to power the headstages/Avatar units, conducting the recorded data through a custom made tether cable from our animals. A six camera Bluecherry digital video recording system is able to record the six separate units and be accessed remotely. Each housing unit is equipped with a custom designed rat running wheel. Activity of these running wheels can be monitored and recorded remotely using Clock Lab software. EDF browser grants us the ability to visualize our electrophysiology recordings on site or remotely in real time. Effectively this system is able to record and monitor local field potential electrophysiology, animal behaviour and wheel running activity of six animals for extremely long periods of time with complete remote access.